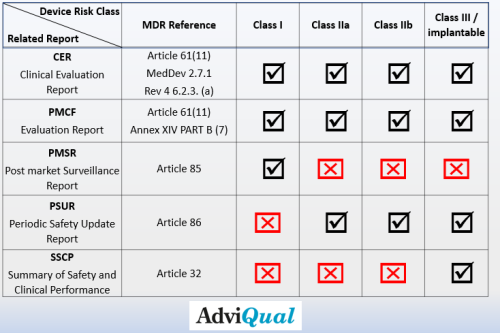
Manufacturers should periodically prepare and keep up-to-date reports listed below to meet MDR requirements and demonstrate product conformity.
- Clinical Evaluation Report (CER)
- Post-Market Clinical Follow-up Report (PMCF - Evaluation Report)
- Post-Market Surveillance Report (PMSR)
- Periodic Safety Update Report (PSUR)
- Summary of Safety and Clinical Performance (SSCP)
Clinical Evaluation Report (CER)
According to article 61 (11) of the MDR (2017/745), the clinical evaluation and its documentation shall be updated throughout the life cycle of the device concerned with clinical data obtained from the implementation of the manufacturer's PMCF plan in accordance with Part B of Annex XIV and the post-market surveillance plan referred to in Article 84.
The manufacturers are expected to evaluate, report and keep the clinical data up-to-date for all product classes (Class I, IIa, IIb and III). The Clinical Evaluation Report (CER) is a report that should be prepared for all product classes and the update frequency of which will depend onthe risk classification and configuration level as well as the technological innovation of the product.
We recommend that CER be updated at least once a year for Class III and implantable devices and at most every two years for other classes.
Post-Market Clinical Follow-up Report (PMCF - Evaluation Report)
As per Annex XIV Part B (7), the manufacturer shall analyse the findings of the PMCF and document the results in a PMCF evaluation report that shall be part of the clinical evaluation report and the technical documentation.
We would like to underline that we will often be reminded that this type of clinical data reporting is an integral part of our lives. The Post-Market Clinical Follow-up (PMCF) Report is prepared for all product classes (Class I, IIa, IIb and III). For cases where it is not necessary, it should be justified.
Post-Market Surveillance Report (PMSR)
In accordance with Article 85 of the MDR, manufacturers of class I devices shall prepare a post-market surveillance report summarising the results and conclusions of the analyses of the post-market surveillance data gathered as a result of the post-market surveillance plan referred to in Article 84 together with a rationale and description of any preventive and corrective actions taken. The report shall be updated when necessary and made available to the competent authority upon request.
No Post-Market Surveillance Report (PMSR) is required for class IIa, class IIb, and class III devices. The above mentioned results for products belonging to these risk classes are presented in the Periodic Safety Update Report (PSUR).
Periodic Safety Update Report (PSUR)
According to MDR Article 86; manufacturers of class IIa, class IIb and class III devices shall prepare a periodic safety update report (‘PSUR’) for each device and where relevant for each category or group of devices summarising the results and conclusions of the analyses of the post-market surveillance data gathered as a result of the post-market surveillance plan referred to in Article 84 together with a rationale and description of any preventive and corrective actions taken.
The update periods of PSUR according to product classes and publication methods are given in the table below.
| Device Class | Update Period | Manufacturer Report Release Method |
|---|---|---|
| Class IIa | When necessary and at least biannually | PSURs are made available to the notified body involved in the conformity assessment and, upon request, to the competent authorities. |
| Class IIb | at least annually | PSURs are made available to the notified body involved in the conformity assessment and, upon request, to the competent authorities. |
| Class III / Implantable Medical devices | at least annually | PSUR should be presented to the notified body involved in the conformity assessment via the electronic system referred to in article 92 of the MDR. The notified body examines the report and adds its own assessment to this electronic system along with the details of each action taken. Such PSURs and notified body assessments are made available to competent authorities through this electronic system. |
Summary of Safety and Clinical Performance (SSCP)
In accordance with article 32 of the MDR, for implantable devices and for class III devices, other than custom-made or investigational devices, the manufacturer shall draw up a summary of safety and clinical performance.
The Summary of Safety and Clinical Performance shall be written in a way that is clear to the intended user and, if relevant, to the patient, and shall be made available to the public via Eudamed.
The draft summary of safety and clinical performance shall be part of the documentation to be submitted to the notified body involved in the conformity assessment pursuant to Article 52 and shall be validated by that body. After its validation, the notified body shall upload the summary to Eudamed. The manufacturer shall mention on the label or the instructions for use where the summary is available.
The Summary of Safety and Clinical Performance (SSCP) should be designed to provide an accurate understanding of the risks and benefits of medical devices for both healthcare professionals and patients. The SSCP should be kept up to date in EUDAMED, and when the PMCF evaluation report and the Periodic Safety Update Report (PSUR) are updated at least annually, the summary (SSCP) should be reviewed and updated if necessary to ensure that the clinical and/or safety information is accurate and complete.