A guide has been published by the Medical Device Coordination Group (MDCG) for implant cards that closely concern medical device manufacturers. Below is a summary of therelevant sections of the MDCG 2019-8 Guideline which we believe will be useful to you.
The purpose of preparing and delivering the Implant Card, given to patients together with Implant Medical Devices is as follows:
- Enabling the patient to identify implanted devices and access other implant-related information (eg. via EUDAMED and/or other websites)
- Allowing patients to identify themselves as people in need of special care when necessary (such as security checks)
- Activation, eg. providing information on patients’ required special care/needs to emergency clinical staff or first responders in case of emergencies.
The legal articles regarding the Medical Device Regulation for Implant Card Design are as follows. Remember to read the following articles of the Medical Device Regulation (MDR) before creating implant cards for your products.
| Article 18 | Mentions the delivery of the implant card with the product by the manufacturer. |
|
Article 18 Paragraph 1a |
Contains the information that should be placed on the implant card by the manufacturer. |
|
Article 18 Paragraph 2 |
Sets out the responsibilities of the Member States regarding their request from health institutions to issue an implant card to the patient. |
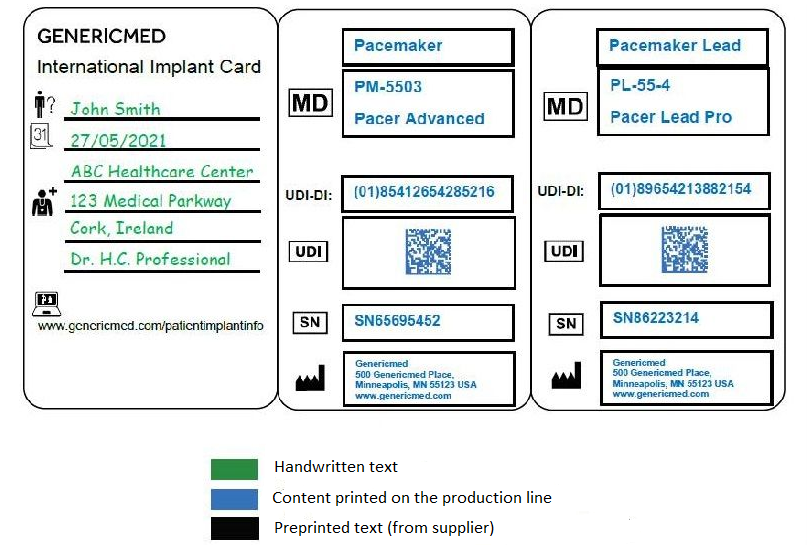
Implant Card Design
Contents:
According to Article 18 paragraph 1a of the MDR, manufacturers should provide the following information on the implant card. This information should preferably be printed on the card, or alternatively, can be added in the clinician-affixed labels.
- Device name
- Serial number, lot number
- UDI number
- Manufacturer’s name, address, website
- Product type (such as a pacemaker, hip prosthesis)
Article 18, paragraph 2 sets out the responsibilities of the Member States regarding their request from health institutions to issue an implant card to the patient concerned. For this reason, there should be blank fields where the patient's identity information can be filledin.
Member States will ensure that only the following information is required within the context of national implementation of Article 18 of the MDR since many different national implant card designs can be very expensive due to the logistics behind preparing them and have no additional benefits.
- Patient’s name or patient ID
- Name and address of the healthcare institution or healthcare provider
- Implantation date
Dimension:
To suit the purposes described in Article 18, the external dimensions of the implant card must be the same as those of a credit card, ATM card or ID card (85.60 mm × 53.98 mm and 2.88–3.48 mm in radius) (Reference: ISO/IEC 7810 ID-1).
The text provided on the implant card by the health institution or healthcare provider and on the completion instruction must be legible and at least 2 millimeters high.
Use of Symbols:
It is recommended to use symbols rather than creating different versions of the implant card for each country. An explanation of the symbols on the implant card should be found in a booklet or, if space permits, on the back of the implant card.
Symbols that can be used are given in the MDCG 2019-8 Guideline.
It is expected that a symbol will be prepared for the definition of "Device Type".
You can access samples for the implant card and explanation booklet from the MDCG 2019-8 Guidance.